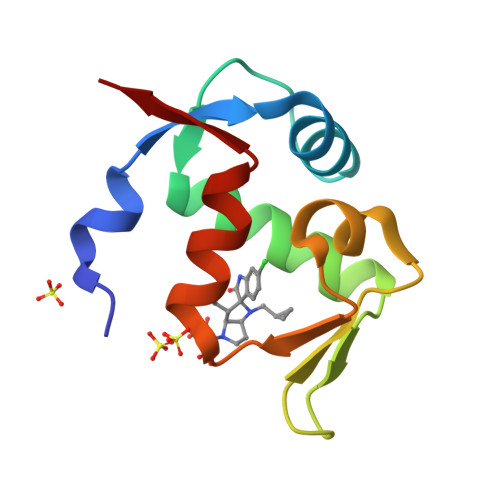
Discovery of Novel Spiro[3H-indole-3,2'-pyrrolidin]-2(1H)-one Compounds as Chemically Stable and Orally Active Inhibitors of the MDM2-p53 Interaction.
Gollner, A., Rudolph, D., Arnhof, H., Bauer, M., Blake, S.M., Boehmelt, G., Cockroft, X.L., Dahmann, G., Ettmayer, P., Gerstberger, T., Karolyi-Oezguer, J., Kessler, D., Kofink, C., Ramharter, J., Rinnenthal, J., Savchenko, A., Schnitzer, R., Weinstabl, H., Weyer-Czernilofsky, U., Wunberg, T., McConnell, D.B.(2016) J Med Chem 59: 10147-10162
- PubMed: 27775892
- DOI: https://doi.org/10.1021/acs.jmedchem.6b00900
- Primary Citation of Related Structures:
5LAV, 5LAW, 5LAY, 5LAZ - PubMed Abstract:
Scaffold modification based on Wang's pioneering MDM2-p53 inhibitors led to novel, chemically stable spiro-oxindole compounds bearing a spiro[3H-indole-3,2'-pyrrolidin]-2(1H)-one scaffold that are not prone to epimerization as observed for the initial spiro[3H-indole-3,3'-pyrrolidin]-2(1H)-one scaffold. Further structure-based optimization inspired by natural product architectures led to a complex fused ring system ideally suited to bind to the MDM2 protein and to interrupt its protein-protein interaction (PPI) with TP53. The compounds are highly selective and show in vivo efficacy in a SJSA-1 xenograft model even when given as a single dose as demonstrated for 4-[(3S,3'S,3'aS,5'R,6'aS)-6-chloro-3'-(3-chloro-2-fluorophenyl)-1'-(cyclopropylmethyl)-2-oxo-1,2,3',3'a,4',5',6',6'a-octahydro-1'H-spiro[indole-3,2'-pyrrolo[3,2-b]pyrrole]-5'-yl]benzoic acid (BI-0252).
Organizational Affiliation:
Boehringer Ingelheim RCV GmbH & Co. KG , Dr. Boehringer-Gasse 5-11, A-1121 Vienna, Austria.